We revolutionizes the effectiveness of inhaled drugs
Excipient technology
Aquilon Pharma’s excipient technology improves both the pharmacology and the lung deposition of existing drugs. It also allows to make other, known drugs inhalable.
A formulation platform for both liquid formulations (solutions) and dry powders
AQ001S and AQ002P are Aquilon Pharma’s first innovative products. The excipient technology makes Corticosteroids soluble in water and suitable for dry powder formulations.
Compatibility
Aquilon Pharma’s excipient technology makes all liquid formulations for nebulization compatible with all types of nebulizers.
Golf ball effect
The excipient/active complex creates fine particles, either in a soft mist or transformed into a dry powder, with an aerodynamic form, including the active ingredient. This ensures drug deposition in the deep lung, where maximal bioavailability is guaranteed.
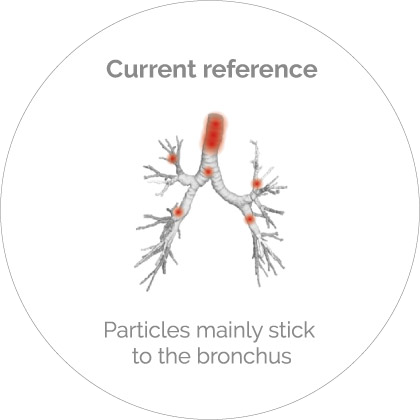
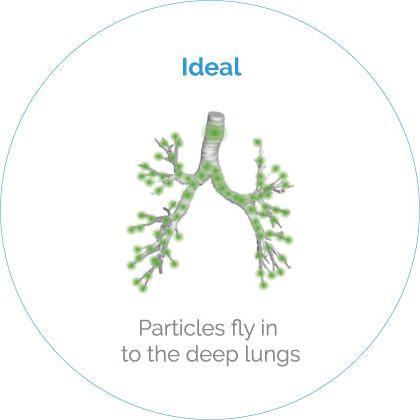
HP-Betadex is the basis of a formulation platform for all compounds, both as solutions and dry powders. Combined with innovative devices, the efficacy improves by a factor 5*
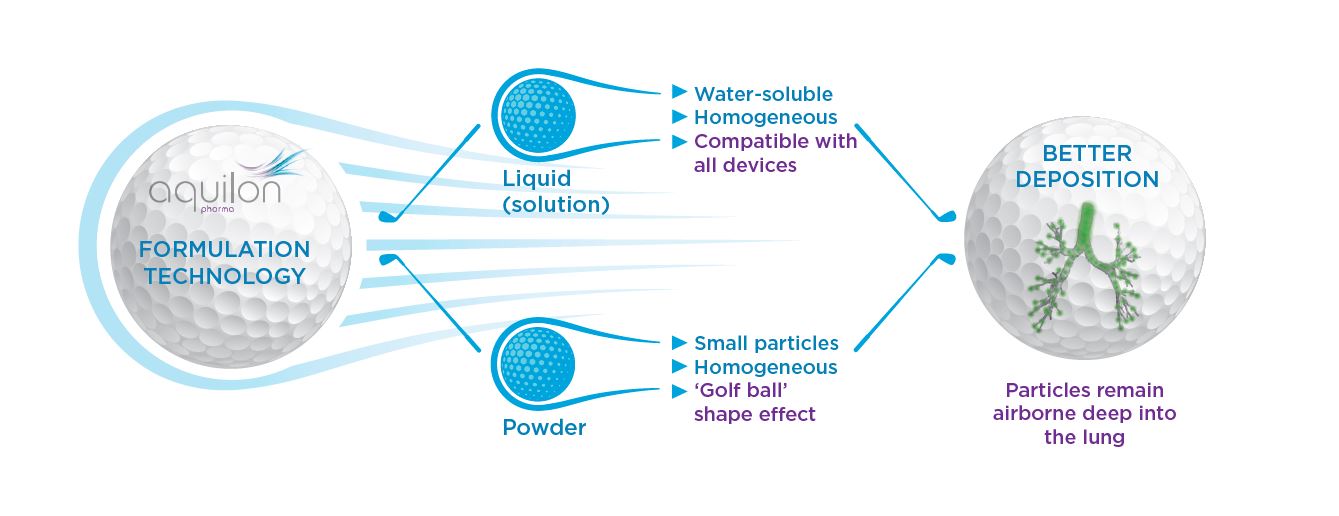
Aquilon offers the opportunity to improve and extend the lifecycle of a broad range of existing drugs
References
- Evrard B, Bertholet P, Gueders M, et al. Cyclodextrins as a potential carrier in drug nebulization. J Control Release 2004; 96:403–410.
- Bertholet P, Gueders M, Dive G, et al. The effect of cyclodextrins on the aqueous solubility of a new MMP inhibitor: phase solubility, 1 H-NMR spectroscopy and molecular modeling studies, preparation and stability study of nebulizable solutions. J Pharm Pharm Sci 2005; 8:163–74. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16124927
En savoir plus
- Evrard B, Bertholet P, Gueders M, et al. Cyclodextrins as a potential carrier in drug nebulization. J Control Release 2004; 96:403–410.
- Bertholet P, Gueders M, Dive G, et al. The effect of cyclodextrins on the aqueous solubility of a new MMP inhibitor: phase solubility, 1 H-NMR spectroscopy and molecular modeling studies, preparation and stability study of nebulizable solutions. J Pharm Pharm Sci 2005; 8:163–74. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16124927.
- Piel G, Piette M, Barillaro V, Castagne D, Evrard B, Delattre L. Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int J Pharm 2007; 338:35–42. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17289314.
- Gueders MM, Bertholet P, Perin F, et al. A novel formulation of inhaled doxycycline reduces allergen-induced inflammation, hyperresponsiveness and remodeling by matrix metalloproteinases and cytokines modulation in a mouse model of asthma. Biochem Pharmacol 2008; 75:514–26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17950252.
- Salem LB, Bosquillon C, Dailey LA, et al. Sparing methylation of β-cyclodextrin mitigates cytotoxicity and permeability induction in respiratory epithelial cell layers in vitro. J Control Release 2009; 136:110–116. Available at: http://dx.doi.org/10.1016/j.jconrel.2009.01.019.
- Rocks N, Bekaert S, Coia I, et al. Curcumin-cyclodextrin complexes potentiate gemcitabine effects in an orthotopic mouse model of lung cancer. Br J Cancer 2012; 107:1083–92. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22929882.
- Dufour G, Bigazzi W, Wong N, et al. Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int J Pharm 2015; 495:869–878. Available at: http://dx.doi.org/10.1016/j.ijpharm.2015.09.052.
- Dufour G, Evrard B, de Tullio P. 2D-Cosy NMR Spectroscopy as a Quantitative Tool in Biological Matrix: Application to Cyclodextrins. AAPS J 2015; 17:1501–10. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26304859.
- Dufour G, Evrard B, de Tullio P. Rapid quantification of 2-hydroxypropyl-β-cyclodextrin in liquid pharmaceutical formulations by (1)H nuclear magnetic resonance spectroscopy. Eur J Pharm Sci 2015; 73:20–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25797290.
- Dos Santos AG, Bayiha JC, Dufour G, et al. Changes in membrane biophysical properties induced by the Budesonide/Hydroxypropyl-β-cyclodextrin complex. Biochim Biophys acta Biomembr 2017; 1859:1930–1940. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28642042.
- Rocks N, Vanwinge C, Radermecker C, et al. Ozone-primed neutrophils promote early steps of tumour cell metastasis to lungs by enhancing their NET production. Thorax 2019; 74:768–779. Available at: http://www.ncbi.nlm.nih.gov/pubmed/31142617.
Contact us
Do you have a question? Let's get in touch.




